Chemistry riddle: Water that freezes at above 0 degrees Celsius.
- Thread starter Jang
- Start date
Recommended Videos
Wouldn't adulterants just cause freezing point depression? Pretty sure the OP is fucked here if he wants to use STP.Imthatguy said:Does it have to be chemically pure?
One of the ways professors can tell you weren't paying attention with your melt temps is if you write it as being higher than it should be.
I supposed you could try Mars. You'd still be under "normal atmospheric conditions".
I was thinking of causing an endothermic reaction in the water but I suppose that would bring the temp below 0 Celsius rather than cause freezing above that.Dags90 said:Wouldn't adulterants just cause freezing point depression? Pretty sure the OP is fucked here if he wants to use STP.Imthatguy said:Does it have to be chemically pure?
One of the ways professors can tell you weren't paying attention with your melt temps is if you write it as being higher than it should be.
...
Ok, the OP specifies normal atmospheric conditions, but does that include normal gravity and/or acceleration?
Ok, the OP specifies normal atmospheric conditions, but does that include normal gravity and/or acceleration?
Simple.Jang said:For reasons I think would bore people I need water that freezes at a temperature that is above 0 degrees Celsius. It would be preferable if it was under normal atmospheric conditions.
Thanks in advance
Under normal atmospheric conditions, normal gravity and general normal circumstances, unadulterated water does not freeze over 0° C
Why do you need water freezing at such temperatures anyways?
I seem to recall water having what's known as the 'Triple Point', which is the temperature where water can exist in all three states. This temperature is 0.01 degrees, so I suppose I'll go with this answer.
Saw this on another site somewhere. I don't know if it actually works or if said solution is even readily available.
Dissolve ammonia based fertilizer in water until it is at solution. Place fresh water in a metal container and rest it in the larger container of fertilizer water and ice will form.
Dissolve ammonia based fertilizer in water until it is at solution. Place fresh water in a metal container and rest it in the larger container of fertilizer water and ice will form.
Under STP not really, the hydrogen bonds in the water crystals aren't that strong and anything above 0 tends to break them. You can add long chain alcohols, which promotes water crystal formation, but the difference is small.
Dissolving NH4 in water is endothermic so all that is doing is refrigerating the water down to 0.
Galaxialconda said:Saw this on another site somewhere. I don't know if it actually works or if said solution is even readily available.
Dissolve ammonia based fertilizer in water until it is at solution. Place fresh water in a metal container and rest it in the larger container of fertilizer water and ice will form.
Dissolving NH4 in water is endothermic so all that is doing is refrigerating the water down to 0.
There is no chemical answer to your question. If you add anything that will allow that freezing to occur at room temperature, it would change the water enough that it wouldn't be water anymore. If you want to freeze water above its freezing point within this planetary atmosphere you need to place it into a perfect vacuum.
Ok, I take it you meant the equilibrium shifts so that it freezes at a greater rate than melting? Because freezing/melting, boiling/condensing happen to an extent at the same time due to the difference in energies of the molecules of water. However, if this is not what you're alluding to, it could be potentially possible to reduce the temperature of the water with a light breeze over the surface to evaporate the higher energy particles and reduce the overall energy of the system.Jang said:For reasons I think would bore people I need water that freezes at a temperature that is above 0 degrees Celsius. It would be preferable if it was under normal atmospheric conditions.
Thanks in advance
Pretty much this: it's a question of equilibira, though (!) water exists at all 3 states at a massive array of temperatures, so, King of Asgard, you need to use the words "in equilibrium" after "all three states", and the temperature is ever so slightly lower (a few more decimal places, but near as makes no real difference).King of Asgaard said:I seem to recall water having what's known as the 'Triple Point', which is the temperature where water can exist in all three states. This temperature is 0.01 degrees, so I suppose I'll go with this answer.
If none of this works for you, then it's because your system is impossible: getting a body of water to freeze under standard conditions (aside from the temperature, which is below standard and above freezing) cannot be done.
Source: PhD chemistry student
Thanks for clarifying.Maeta said:snip
To be honest, I only know about it in very vague terms as I've not studied chemistry, so I didn't know about the terms betwixt your inverted commas.
This might help. There are exotic forms of Ice with different properties, arising from different crystal structure. It's possible to get these at high temperatures if you have high pressure as well.
From Wikipedia:
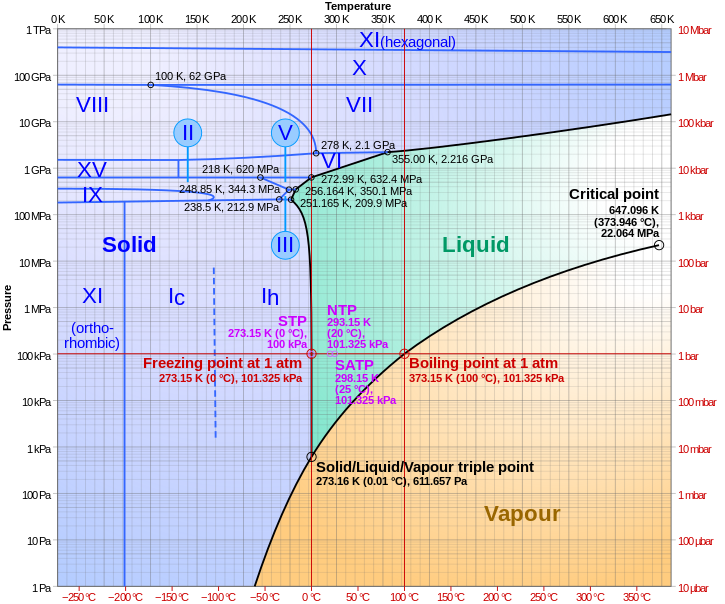
Your best bet is this one (also from wikipedia);
From Wikipedia:

Your best bet is this one (also from wikipedia);
So yeah, if you don't mind working at over 10,000 atmospheres, then it is possible.Ice VI: A tetragonal crystalline phase. Formed by cooling water to 270 K at 1.1 GPa. Exhibits Debye relaxation.[49]
It's no worry: some of the people I've worked around the last 4 years have had trouble with the concept, and some of them are carrying on into PhDs as well.King of Asgaard said:Thanks for clarifying.Maeta said:snip
To be honest, I only know about it in very vague terms as I've not studied chemistry, so I didn't know about the terms betwixt your inverted commas.
Back to the discussion: I remembered a little while ago the appearance of the phase diagram for water, and to promote ice formation due to pressure increase, you need staggeringly high pressures compared to expectations due to the nature of the hydrogen bonding: the first (I think) 3 phases of ice (maybe more) are less dense than water (without going into the elevated phase numbers of ice, the densest water gets is in liquid form at 4 degrees celsius). The higher numbered phases can be achieved at greater than room temperature, but only with incredible pressure, and to even form them below the standard freezing point generally requires crushing pressures. Hope this adds a new dimension to the thinking as another perspective: ice is not just one form, it has many... Like Seven Force from Gun Star Heroes only not as likely to attack you (not only am I a chemist, I'm also bloody weird).
Edit: and as I was typing, I got ninja'd by the diagram... ClockworkPenguin, I will come for you...
